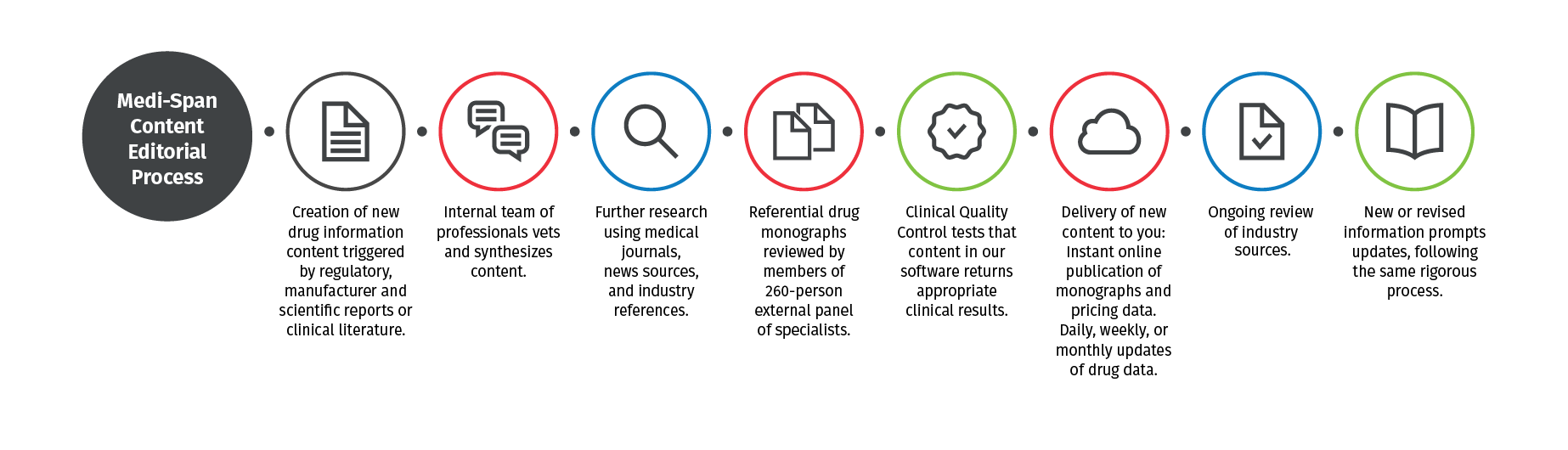
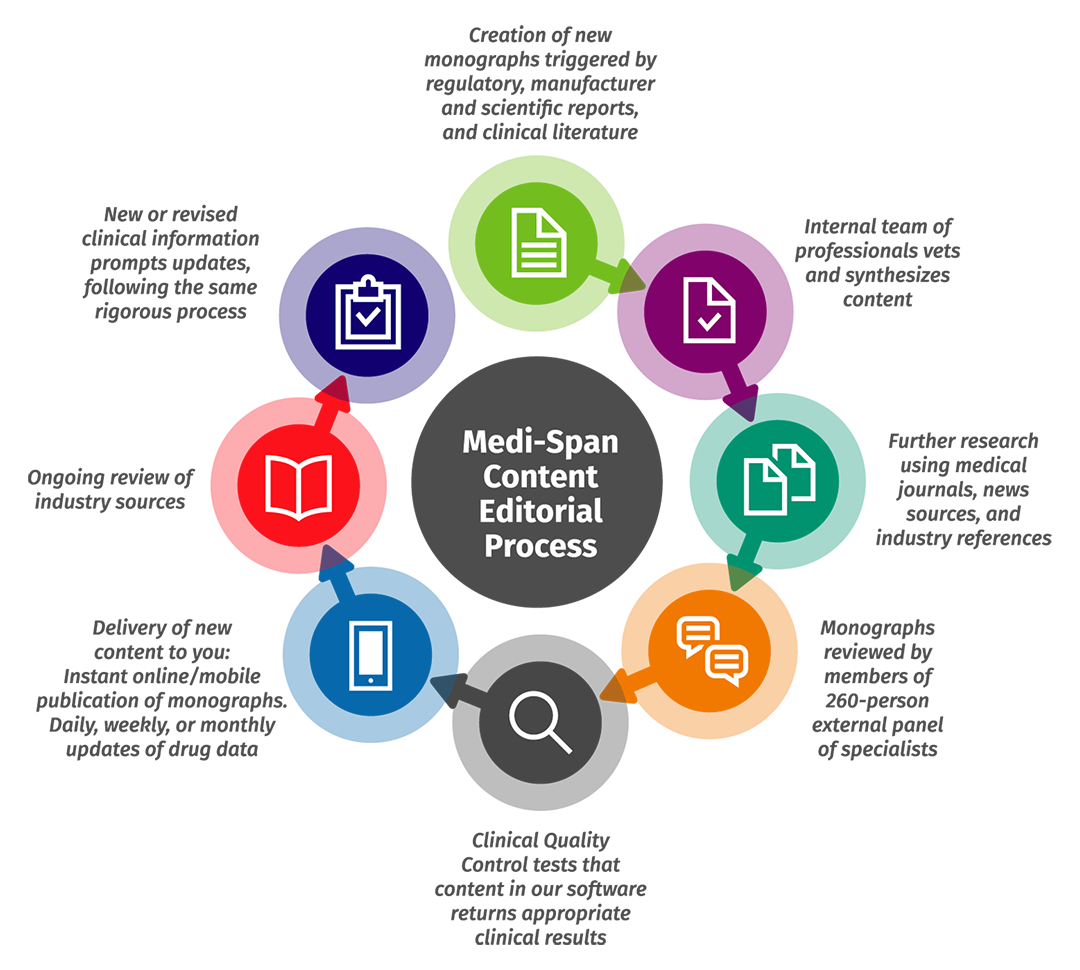
2. Keep pace with changes in the field — without sacrificing rigor.
We balance the need to review and validate information with the need to update content as promptly as possible. Our multidisciplinary team – comprising nearly 150 advanced-degreed clinicians from across disciplines and fields, including pharmacists, physicians, nurses, and medical technologists – works together to keep content current and follow a rigorous editorial process.
As a customer, you will have access to the latest available drug information and medication safety content. Our proactive monitoring and updates include:
- Daily surveillance of industry activity and primary literature so that our information reflects contemporary medical practices and promotes relevance and clarity of content.
- Weekly research to update information pertaining to medication guides, Risk Evaluation and Mitigation Strategies (REMS), and U.S. drug shortages.
- Ongoing review of pharmaceutical manufacturer announcements and publications to find information on new drug availability, new dosage forms, revisions to contraindications, warnings, drug interactions, labeling changes, and more.
- Internal clinical team review of medical science updates such as published literature within key therapeutic areas, input related to clinical guidelines, and clinical practice